BlueDil International serves as your virtual affiliate, providing a cost-effective approach to expanding your business in the market of rare disease orphan drugs across Europe and beyond. With the expertise of former SOBI executives and a ready-to-deploy, agile, and fully-integrated team, we offer tailored solutions and support to meet your unique business needs.
Navigating Challenges and Seizing Opportunities in the European Rare Disease Market
Market conditions in Europe differ across countries, as do cultures and languages. The regulatory landscape is complex and rapidly changing, shaped by country-specific regulations. Navigating this intricate ecosystem requires agility and a deep understanding of diverse regional requirements.
A centralized market authorization process, yet 28 different National Health Systems.
More than 50 Medicine Regulatory Authorities
More than 20 official spoken languages
At the same time Europe presents unparalleled opportunities for growth and innovation in rare disease and orphan drugs.
More than 30 million European people are living with a rare disease
Early Access Programs (EAPs) approvable in many European countries
Over 230 authorized orphan therapies in Europe
20 to 25 new orphan therapy market authorizations per year
By leveraging our long-lasting European expertise in rare diseases, orphan drugs and niche indications, BlueDil International can help you navigate the complexities of rare disease pharma, to unleash the full potential of your business in Europe.
Our strategic and operational services will support your rare disease company during the products’ lifecycle, from clinical development, to pre-approval and commercialization.
We will develop and enhance your relationships at country and regional level, engaging with all relevant European stakeholders, building your own network of Scientific Societies, KOLs and HCPs, PAGs, Regulatory bodies, Payers and Policymakers.
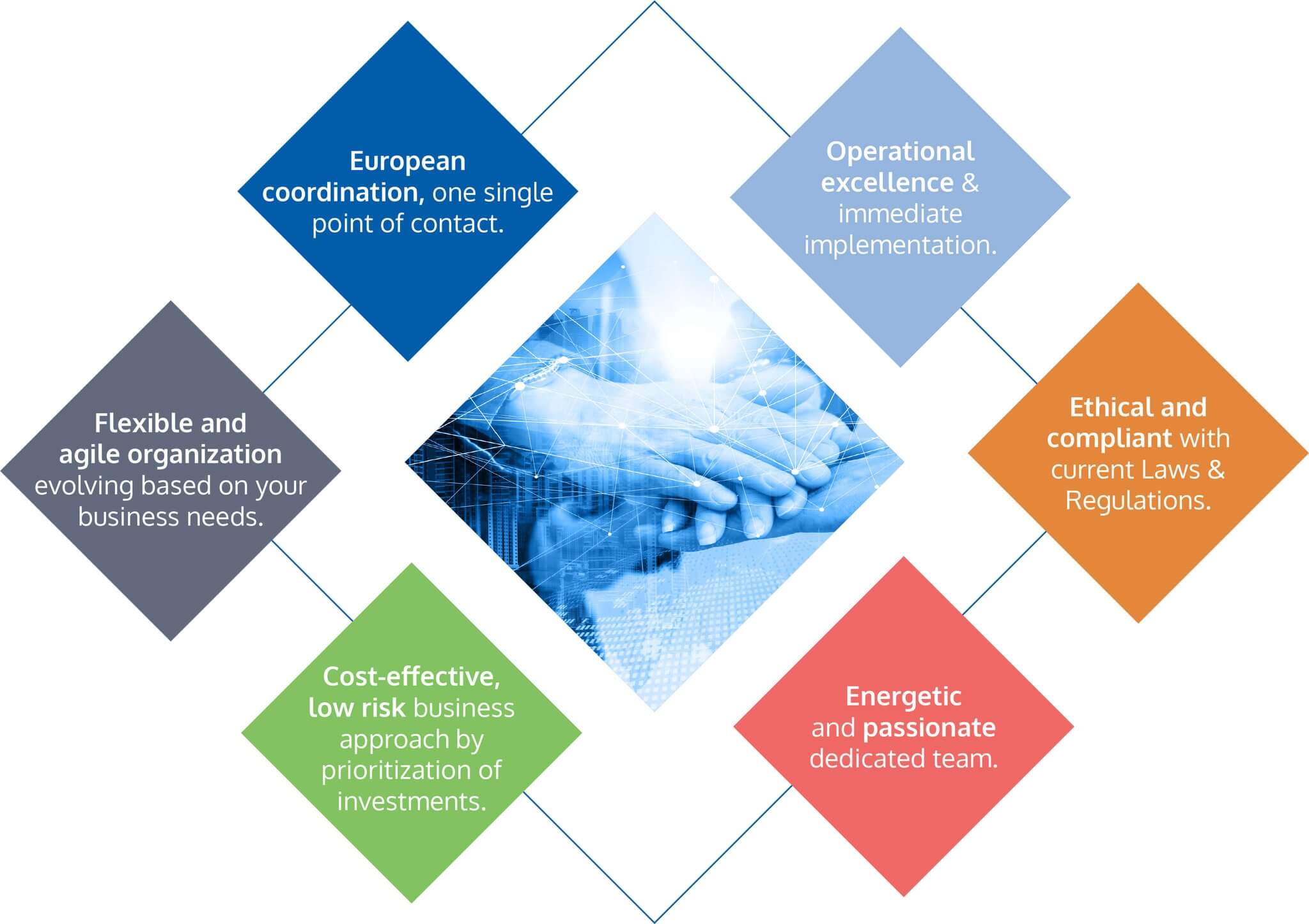
Our customized approach, aimed to reach excellence and success, is key to thriving in this dynamic environment.
Our Excellence, Your Success
Driven by Specialized Knowledge
Driven by Specialized Knowledge
With over 30 years of experience in rare diseases and orphan therapies and more than 1,000 cumulative events, we remain the forefront of scientific, technological, and regulatory advancements. This ensures that BlueDil delivers cutting-edge, tailored solutions that meet the unique needs of each market.
Powered by Extensive Experience
Powered by Extensive Experience
With over 160 cumulative years of experience in the pharmaceutical industry, BlueDil founding partners leverage their extensive and long-standing experience to build a broad and influential network, crucial for establishing strategic collaborations and gathering valuable insights.
Refined by Valuable Expertise
Refined by Valuable Expertise
The BlueDil leadership team is distinguished by its top-tier executive experience, with each senior partner having held key strategic and operational roles in pharmaceutical and biotech companies, specializing in orphan therapies. Our expertise spans regulatory affairs, marketing, and business strategy, providing a comprehensive and integrated consultancy service. Having completed over 50 missions for companies in the rare disease sector, our team is uniquely positioned to provide valuable insights and strategies tailored to your needs.
Achieved through Proven Teamwork
Achieved through Proven Teamwork
With over 15 years of collaboration, BlueDil partners have cultivated a unique synergy that enables us to work seamlessly together. This deep-rooted teamwork empowers us to effectively tackle complex challenges and deliver cohesive solutions.